Title: A Versatile One‐Pot Synthesis of Polysubstituted Cyclopent‐2‐enimines from α,β‐Unsaturated Amides via Imino‐Nazarov Reaction
Author: Ting Fan, Ao Wang, Jia-Qi Li, Jian-Liang Ye, Xiao Zheng, Pei-Qiang Huang
Summary:
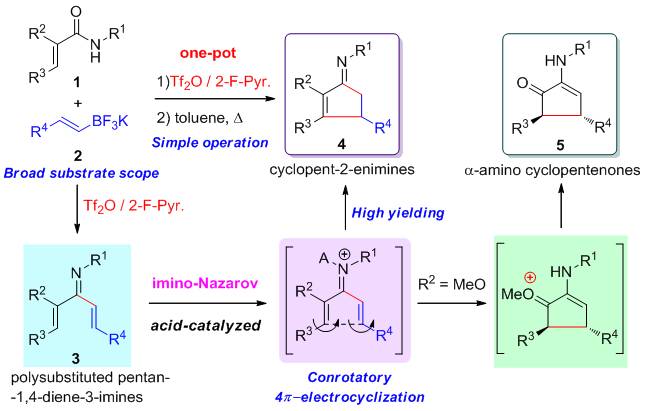
The imino‐Nazarov cyclization of the polysubstituted pentan‐1,4‐diene‐3‐imine is realized. To this aim, a one‐pot procedure involving reductive alkenyliminylation of α,β‐unsaturated secondary amides with potassium organotrifluoroborates followed by acid‐catalyzed imino‐Nazarov cyclization of the polysubstituted pentan‐1,4‐diene‐3‐imine intermediates is studied systematically. This mild, operationally simple, flexible, and high‐yielding protocol efficiently affords polysubstituted pentan‐1,4‐diene‐3‐imines, cyclopentenimines, and α‐amino cyclopentenones, which are useful scaffolds in organic synthesis. The substitution effect at the C‐2 position of polysubstituted pentan‐1,4‐diene‐3‐imines was studied by means of density functional theory calculations. Results suggested that the electron‐donating group facilitates the imino‐Nazarov cyclization process.
Full Link:https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201805641