Prof. Wenjing Hong’s group, in collaboration with researcher in University of Bern and University of Copenhagen, published a joint paperentitled “Single-molecule detection of dihydroazulene photo-thermal reaction using break junction technique” in Nature Communications (Nature communications, 2017, 8, 15436; DOI: 10.1038/ncomms15436).
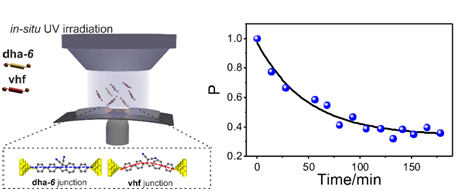
At single-molecule scale, the slight change in the molecular structurewill lead to significant variation in electrical properties,thus it is possible to investigate chemical reaction through single-molecule electrical measurement. In this paper, they explored the potential of the single-molecule break junction technique in the detection of photo-thermal reaction processes of a photochromic dihydroazulene/vinylheptafulvene system. The product ratios observed when switching the system in the junction does not follow those observed in solution studies (both experiment and theory), suggesting that the junction environment was perturbing the process significantly. This study opens the possibility of using nano-structured environments like molecular junctions to tailor product ratios in chemical reactions.
This work was supervised by Prof. Wenjing Hong, and Prof. Mogens B. Nielsen from University of Copenhagen in Denmark, and the theoretical calculations were carried out by Prof. Kurt V. Mikkelsen and Gemma C. Solomon’s groups. Assist. Prof. Yang Yang from Pen-Tung Sah Institute of Micro-Nano Science and Technology from Xiamen Universityparticipated in data analysis and mechanism discussions.
Hong’s group has been devoted to the research of the chemical reaction, molecular assembly and electron transportationat single-molecule scale, and constructed a number of scientific instruments with focus on single-molecule characterization. And they explored the quantum interference effect, electrochemical gating and reaction kinetics at single-molecule level by cooperating withgroups. Since 2015, Hong’s group hasmade a series of progresses: electrochemical gating for single Naphthalenediimide molecule (Angew. Chem. Int. Ed., 2015, 54(46): 13586), collaborated with Prof. Deqing Zhang from Institute of Chemistry, CAS; fabrication and characterization of quadruple-hydrogen-bond-bridged supramolecular junction(Angew. Chem. Int. Ed., 2015, 54(46): 13586), collaborated with Prof. Dong Wang and Prof. Yuwu Zhong from Institute of Chemistry, CAS; and gating of quantum interference by heteroatom substitution(Angew. Chem. Int. Ed., 2017, 56(1): 173), collaborated with Prof. Colin Lambert from Lancaster University and Dr. Shixia Liu from University of Bern.
The work in Xiamen was supported by the National Natural Science Foundation of China (No. 21673195, 21503179), and the State Key Laboratory of Physical Chemistry of Solid Surfaces, and Collaborative Innovation Center of Chemistry for Energy Materials.
Full Link: https://www.nature.com/articles/ncomms15436