Title: Fenton-Derived OH Radicals Enable the MPnS Enzyme to Convert 2-Hydroxyethylphosphonate to Methylphosphonate: Insights from Ab Initio QM/MM MD Simulations
J. Am. Chem. Soc. 2019, 141, 23, 9284-9291
Authors:
Binjun Wang*, Zexing Cao, Carme Rovira, Jinshuai Song, Sason Shaik*
Abstract:
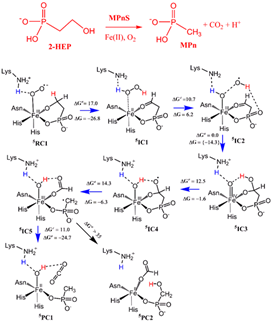
The mechanism for dioxygen activation represents one of the core issues in metalloenzymes. In most cases, the activation of the O2 molecule requires additional electrons from an external reducant. However, nonheme hydroxyethylphosphonate dioxygenase (HEPD) and methylphosphonate synthase (MPnS) are exceptional C–H oxygenases. Both enzymes do not utilize reductants, rather they employ directly iron(III)-superoxide species to initiate H-abstraction reactions and lead thereby to catalysis of the C–C cleavage in 2-hydroxyethylphosphonate (2-HEP). Using the recently characterized MPnS structure and QM(B3LYP)/MM-based metadynamics simulations, we deciphered the chemical mechanism for MPnS. Our simulations demonstrate O2 activation in MPnS is mediated by an adjacent Lysine residue (Lys28) in the active site, leading to an unusual H2O2intermediate in the reductant-independent nonheme MPnS enzyme. Furthermore, the so-generated H2O2intermediate is subsequently employed in a Fenton-type reaction, leading to a locked •OH radical that spontaneously attaches to the substrate carbonyl group. Meanwhile, the proton from the Fe(III)–OH is shuttled back to the deprotonated Lys28, affording the Fe(IV)-oxo species that is identified by experiment in HEPD. Thus, our calculations demonstrate an unusual proton-shuttle mechanism for O2activation in metalloenzymes.
Published: 27 May 2019
Full Link: https://pubs.acs.org/doi/abs/10.1021/jacs.9b02659