Title:Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes
Nat. Catal. 2019, 2, 1016-1026
Authors:
Yanjun Li, Meng Lei, Lei Gong*
Abstract:
The selective functionalization of inert C(sp3)–H bonds is extremely attractive in organic synthesis and catalysis science, but the conversion of hydrocarbons lacking directing groups into chiral molecules through catalytic C(sp3)–H functionalization is formidably challenging. Here, to address this problem, we have developed a photochemical system consisting of a hydrogen atom transfer organophotocatalyst and a chiral catalyst containing an earth-abundant metal. With the cooperative catalysts and imine partners, a wide range of benzylic, allylic hydrocarbons and unactivated alkanes can be converted to functionalized chiral products. The readily tunable bisoxazoline catalysts of copper or other metals exhibit precise regional recognition and asymmetric induction towards these inert C–H bonds. The reactions are applicable to many compounds including small hydrocarbons, branched alkanes, cycloalkanes and more complex medicinal agents. This method provides an economic and rapid construction of optically active compounds, starting from the most basic chemical feedstocks.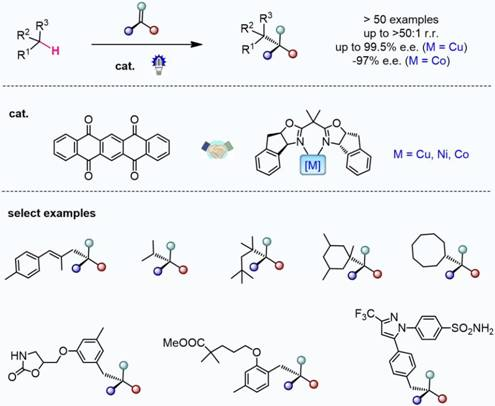
Published: 26 September 2019
Full Link: https://www.nature.com/articles/s41929-019-0357-9