Title:Zinc-Catalyzed Asymmetric Formal [4+3] Annulation of Isoxazoles with Enynol Ethers via 6π Electrocyclization: Stereoselective Access to 2H-Azepines
Angew. Chem. Int. Ed., 2019, 59, 1666-1673
Authors:
Xin-Qo Zhu, Ze-Shu Wang, Bo-Shang Hou, Hao-Wen Zhang, Chao Deng*, Long-Wu Ye*
Abstract:
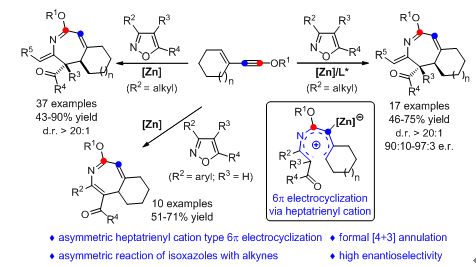
6π electrocyclization has attracted interest in organic synthesis because of its high stereospecificity and atom economy in the construction of versatile 5–7‐membered cycles. However, examples of asymmetric 6π electrocyclization are quite scarce, and have to rely on the use of chiral organocatalysts, and been limited to pentadienyl‐anion‐ and triene‐type 6π electrocyclizations. Described herein is a zinc‐catalyzed formal [4+3] annulation of isoxazoles with 3‐en‐1‐ynol ethers via 6π electrocyclization, leading to the site‐selective synthesis of functionalized 2H‐azepines and 4H‐azepines in good to excellent yields with broad substrate scope. Moreover, this strategy has also been used to produce chiral 2H‐azepines with high enantioselectivities (up to 97:3 e.r.). This protocol not only is the first asymmetric heptatrienyl‐cation‐type 6π electrocyclization, but also is the first asymmetric reaction of isoxazoles with alkynes and the first asymmetric catalysis based on ynol ethers.
Published:13 Novmeber 2019
Full Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.201912534