One-pot cyclization to large peptidomimetic macrocycles by in situ generated β-turn-enforced folding
Authors: Fei Gou, Di Shi, Bohan Kou, Zhao Li, Xiaosheng Yan, Xin Wu, and Yun-Bao Jiang*
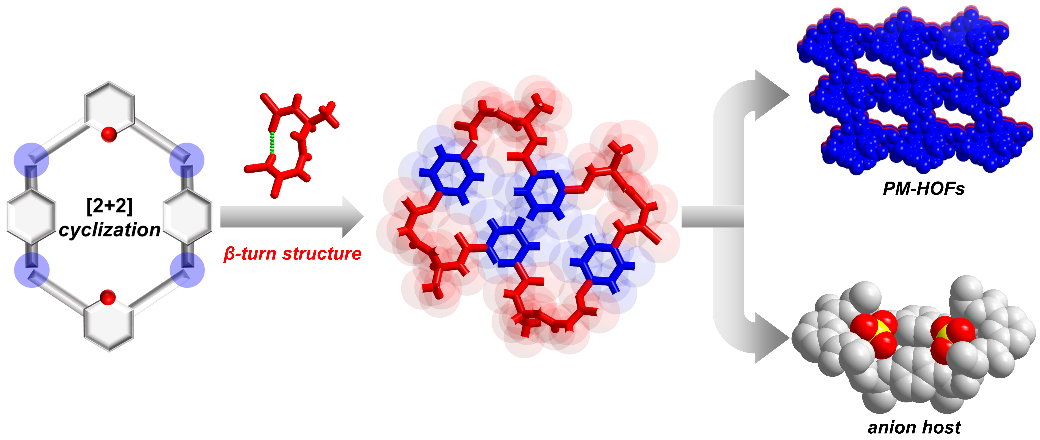
Abstract: Macrocycles have been targets of extensive synthetic efforts for decades because of their potent molecular recognition and self-assembly capabilities. Yet, efficient syntheses of macrocyclic molecules via irreversible covalent bonds remain challenging. Here, we report an efficient approach to large peptidomimetic macrocycles by using the in situ-generated β-turn structural motifs afforded in the amidothiourea moieties from the early steps of the reaction of 2 molecules of bilateral amino acid-based acylhydrazine with 2 molecules of diisothiocyanate. Four chiral and achiral peptidomimetic large macrocycles were successfully synthesized in high yields of 45–63% in a feasible one-pot reaction under sub-molar concentration conditions and were purified by simple filtration. X-ray crystallographic characterization of three macrocycles reveals an important feature that their four β-turn structures, each maintained by four 10-membered intramolecular hydrogen bonds, alternatively network the four aromatic arms. This affords an interesting conformation switching mode upon anion binding. Binding of SO42– to 1L or 1D that contains 4 alanine residues (with the lowest steric hinderance among the macrocycles) leads to an inside-out structural change of the host macrocycle, as confirmed by the X-ray crystal structure of 1L-SO42– and 1D-SO42– complexes, accompanied by an inversion of the CD signals. On the basis of the strong sulfate affinity of the macrocycles, we succeeded in the removal of sulfate anions from water via a macrocycle-mediated liquid–liquid extraction method. Our synthetic protocol can be easily extended to other macrocycles of varying arms and/or chiral amino acid residues; thus, a variety of structurally and functionally diverse macrocycles are expected to be readily made.
Link: https://pubs.acs.org/doi/10.1021/jacs.2c11684