Title: Helical Quintulene: Synthesis, Chirality, and Supramolecular Assembly
Authors: Ling-Xi Huang, Hong-Rui Pan, Jiang-Feng Xing, Dr. Xin-Jing Zhao, Ze-Jia Li, Rong-Jie Xie, Yu-Huan Geng, Qing-Song Deng, Prof. Dr. Yuan-Zhi Tan*
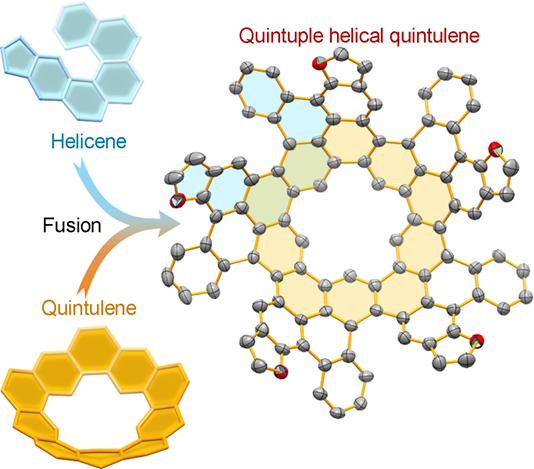
Abstract: Quintulene is a quintuply symmetrical cycloarene with a positively curved molecular geometry. First described by Staab and Sauer in 1984, its successful synthesis was not achieved until 2020. Due to the challenges posed by its positive curvature, structural extensions of quintulene have been studied rarely. In this work, we report the synthesis of a chiral quintulene (1) featuring five [5]helicene units at its rim constructed through the introduction of steric hindrance. Single-crystal X-ray diffraction analysis confirmed the structure of 1 that showcases a unique chirality combining helicity and rotational symmetry. The enantiomers of 1 were separated and their chiroptical properties were examined, revealing the dissymmetric absorption and luminescence. Additionally, the bowl shape facilitates supramolecular assembly between 1 and fullerenes, with a binding constant to C60 10-fold higher than to C70. This work expands the structural diversity of quintulene and bridges the structures of cycloarenes and multi-helicenes, paving the way for the structural design of chiral cycloarenes.
Full-Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202424991