Title: Enantioselective Alkyl–Acyl Radical Cross-Coupling Enabled by Metallaphotoredox Catalysis
Authors: Tao Li, Zhen Xu, Yongliang Huang, Weisai Zu, Haohua Huo*
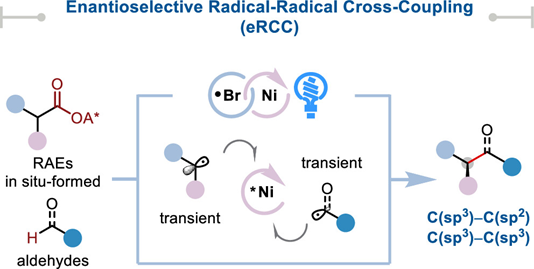
Abstract: Radical–radical cross-coupling (RCC) offers a promising approach for carbon–carbon bond formation in organic synthesis, particularly for creating complex, three-dimensional molecules. However, achieving both cross- and enantioselectivity in RCC reactions has remained a significant challenge. Here, we report a novel metallaphotoredox platform that enables highly enantioselective decarboxylative coupling of carboxylic acid derivatives with aldehydes. Our strategy leverages independent control over radical generation and subsequent enantioselective bond formation through fine-tuning of a common photocatalyst and a simple chiral bis(oxazoline) nickel catalyst. This redox-neutral protocol requires no exogenous oxidants or reductants and demonstrates broad substrate scope and functional group compatibility in the synthesis of enantioenriched α-aryl and α-amino ketones. The α-amino ketone products can be readily transformed into valuable β-amino alcohols, streamlining access to these important motifs. Furthermore, we showcase the potential of this approach for more challenging enantioselective C(sp3)–C(sp3) alkyl–alkyl RCC reactions. This unified platform for enantioselective alkyl–acyl radical cross-coupling represents a significant advance in asymmetric catalysis and underscores the potential for metallaphotoredox catalysis to exploit new mechanisms to solve long-standing synthetic problems.
Full-Link: https://pubs.acs.org/doi/10.1021/jacs.4c15275