Title: Motif-Directed Oxidative Folding to Design and Discover Multicyclic Peptides for Protein Recognition
Authors: Chuanliu Wu*
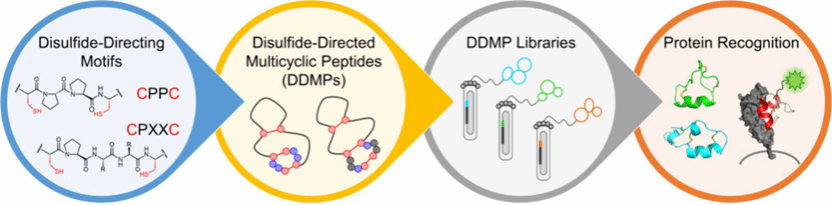
Abstract: Multicyclic peptides that are constrained through covalent cross-linkers can usually maintain stable three-dimensional (3D) structures without the necessity of incorporating noncovalently interacting cores. This configuration allows for a greater utilization of residues for functional purposes compared to larger proteins, rendering multicyclic peptides attractive molecular modalities for the development of chemical tools and therapeutic agents. Even smaller multicyclic peptides, which may lack stable 3D structures due to limited sequence-driven folding capabilities, can still benefit from the specific conformations stabilized by covalent cross-linkers to facilitate target binding. Disulfide-rich peptides (DRPs) are a class of particularly significant multicyclic peptides that are primarily composed of disulfide bonds in their interior. However, the structural diversity of DRPs is limited to a few naturally occurring and designer scaffolds, which significantly impedes the development of multicyclic peptide ligands and therapeutics. To address this issue, we developed a novel method that utilizes disulfide-directing motifs to design and discover DRPs with new structures and functions in random sequence space. Compared with traditional DRPs, these new DRPs that incorporate disulfide-directing motifs exhibit more precise oxidative folding regarding disulfide pairing and demonstrate greater tolerance to sequence manipulations. Thus, we designated these peptides as disulfide-directed multicyclic peptides (DDMPs).
Full-Link: https://pubs.acs.org/doi/10.1021/acs.accounts.5c00060