Title: Identifying high-spin hydroxyl-coordinated Fe3+N4 as the active centre for acidic oxygen reduction using molecular model catalysts
Authors: Kuang-Min Zhao, Dao-Xiong Wu, Wen-Kun Wu, Jia-Bao Nie, Fu-Shan Geng, Guang Li, Hai-Yan Shi, Sheng-Chao Huang, Huan Huang, Jing Zhang, Zhi-You Zhou, Yu-Cheng Wang* & Shi-Gang Sun*
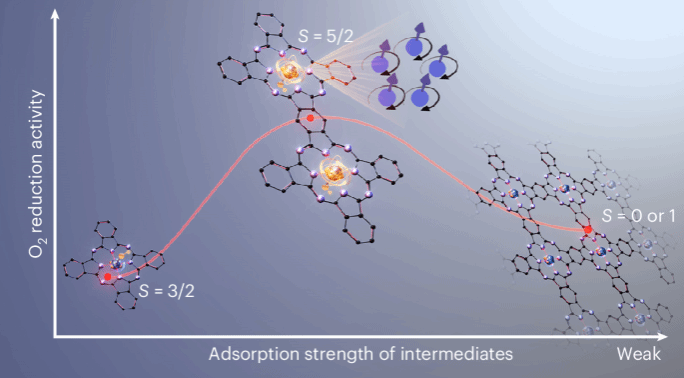
Abstract: Fe–N–C catalysts are the most promising alternative to Pt for the acidic oxygen reduction reaction (ORR), yet the electronic structure of their active centres remains elusive. Here we synthesize and characterize a conjugate-bridged iron phthalocyanine (FePc) dimer model catalyst with identical Fe sites and catalytic activity comparable to actual catalysts. A high-spin trivalent FeN4 with an axial hydroxyl ligand, denoted as OH–Fe3+N4 (S = 5/2), is identified as the active state. By contrast, monomer and non-conjugated dimer manifest the OH–Fe3+N4 (S = 3/2) state with an excessive adsorption energy of ORR intermediates. Polymerized FePc is composed of 35% of the S = 5/2 state and 65% of the Fe2+N4 (S = 0 or 1) state, showing a general weaker adsorption energy. Both overly strong and weak adsorption energy hinder the ORR. Theoretical calculations indicate that π–d interaction between Fe and the conjugated carbon plane dictates the spin state. This study will help to precisely design Fe-based ORR catalysts.
Full-Link: https://www.nature.com/articles/s41929-025-01324-7