Title: Gram-Scale Total Synthesis of Illisimonin A
Authors: Liangchao Zhu, Jinrui Li, Zhaohong Lu*
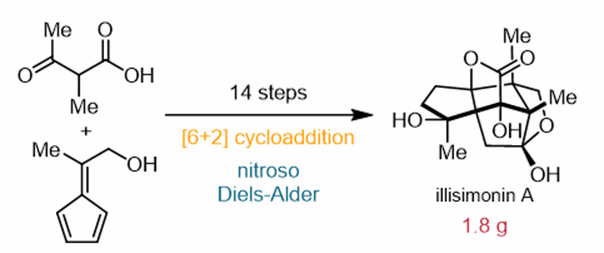
Abstract: Illisimonin A, a structurally complex sesquiterpenoid isolated from the Illicium genus, possesses a 5/5/5/5/5 pentacyclic scaffold featuring seven contiguous, fully substituted chiral quaternary carbon centers. Herein, we report a gram-scale total synthesis of (±)-Illisimonin A achieved in 14 steps. The strategic approach features several key transformations: (1) a pentafulvene-involved intramolecular [6 + 2] cycloaddition that rapidly assembles the linear 5/5/5 tricyclic core, (2) a pentafulvene-involved intramolecular alkylation enabling polycyclic framework construction, (3) a nitroso-Diels–Alder reaction for precise oxidation state installation, and (4) a late-stage Ru-catalyzed oxidative lactonization.
Full-Link: https://pubs.acs.org/doi/10.1021/jacs.5c07921