Title: Direct Reductive Transformation of Amides: From Methodology Development to Applications in Total Synthesis
Authors: Wei Ou, Pei-Qiang Huang*
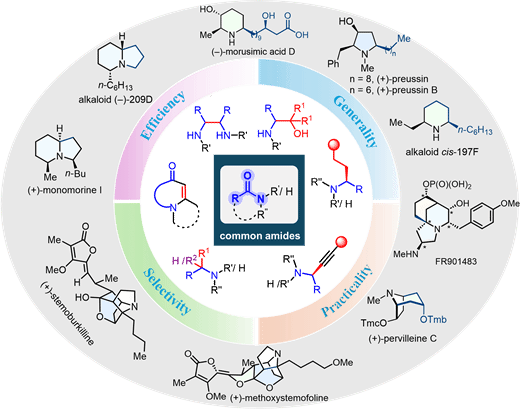
Abstract: Efficiency, selectivity, versatility, and practicality are major goals in current organic synthesis. Carboxamides are among the most prevalent and versatile functional groups in organic and medicinal chemistry, prominently featured in pharmaceuticals, natural products, and biomolecules. A large-scale analysis of over 1 million pharmaceutical reactions revealed that amide formation is the most frequently used transformation, and amine synthesis remains a foundational transformation in medicinal chemistry. Among various approaches, the reductive functionalization of amides has emerged as a particularly powerful strategy, especially in the concise synthesis of nitrogen-containing alkaloids with diverse biological activities.
However, the exceptional stability of amides, due to resonance delocalization, renders them chemically inert toward nucleophilic addition and thus difficult to transform directly. Traditional strategies often require multistep protocols or harsh reagents, thereby limiting their efficiency, selectivity, and compatibility with sensitive functional groups.
Our group has contributed significantly to overcoming these challenges by developing general and chemoselective methods for the direct reductive transformation of amides. Since our initial reports in 2010, we have introduced multiple mechanistic paradigms─including triflic anhydride activation and fully catalytic relay processes─that enable the efficient synthesis of α-functionalized amines and alkaloids. These include direct reductive alkylation, bisalkylation, and more recently, catalytic enantioselective reductive alkynylation and alkylation of both secondary and tertiary amides.
In this Account, we summarize over a decade of progress in this area, focusing on the development of new activation strategies (e.g., Ir–Cu relay catalysis, organocatalyst integration, Pd-mediated tandem transformations), mechanistic understanding, and synthetic applications. We highlight how our catalytic asymmetric methodologies have redefined the utility of amides as building blocks, enabling highly efficient and selective construction of complex nitrogen heterocycles and natural products. Moreover, we discuss the broader implications of these advances in medicinal chemistry and process-scale synthesis. The impact of these methodologies is further demonstrated through their adoption by other research groups in total synthesis and drug development, underscoring their generality and transformative potential.
Full-Link: https://pubs.acs.org/doi/10.1021/acs.accounts.5c00366